The
Situation
FDA Ruling
An FDA rule taking effect in 2023 will mandate that all imaging centers inform women who receive a mammogram about the density of their breasts, the corresponding risks associated with breast tissue density and recommendations for further testing, where applicable.
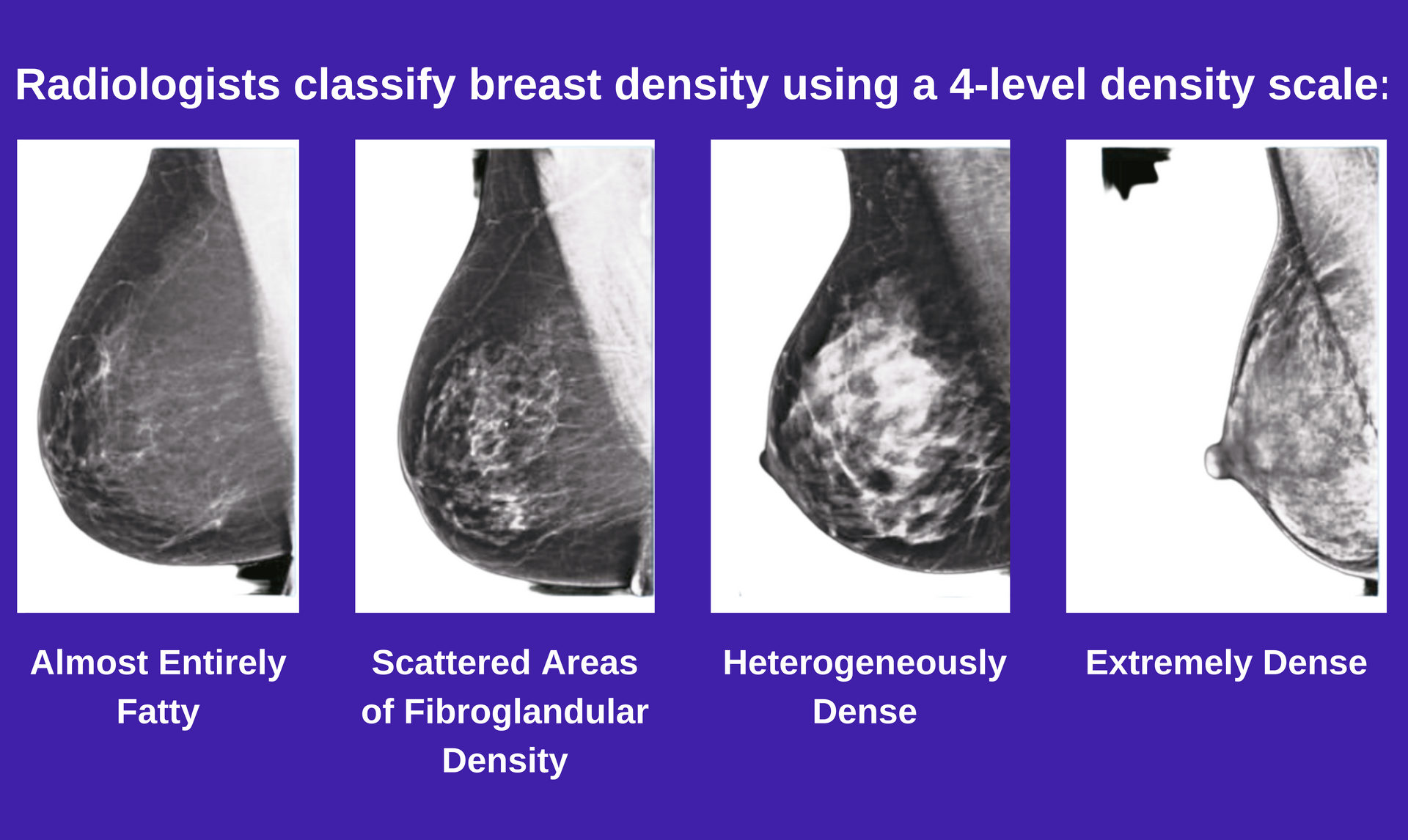
4-Level Density Scale
Radiologists classify breast density using a 4-level density scale, with categories A-D (images left to right) representing the various levels of breast density.
Each year, approximately 56M women receive a mammogram.
- 40% of these women fall within category C (heterogeneously dense).
- 10% of these women fall within category D (extremely dense).
Studies Show
THE 28M WOMEN IN DENSITY CATEGORIES C & D ARE
20 - 400%
MORE LIKELY TO DEVELOP BREAST CANCER COMPARED TO WOMEN WITH LESS DENSE BREASTS
Why so much added risk?
Detection Limitations
A normal mammogram should appear mostly black. If cancer is present, it generally appears as a solid white mass.
Women with dense breasts, however, have excessive fibroglandular tissue, which appears white on mammography.
"Checking for cancer in a patient with dense breasts is like searching for a white spot on a white wall."
Dr. Melissa Durand
Associate Professor at the Yale School of Medicine Department of Radiology and Biomedical Imaging
What this means...
"In a completely fatty breast — so lots and lots of black on the mammogram — we can be as accurate as 98%. But our sensitivity can drop really low — in some studies, even as low as 30% — if you have an extremely, extremely dense breast."
The Recommendation
Patient: Elizabeth Cormier-May
Study Date: 11/18/2022
Study Location: Connecticut
"Your breast composition has been described as:
C. The breasts are heterogenously dense, which may obscure small masses. (ACR BIRADS density Category C)"
"For those patients that have breast composition described as either C. the breast are heterogeneously dense, which may obscure small masses or D. the breast are extremely dense, which lowers the sensitivity of mammography, you may qualify for a supplemental breast screening exam such as ultrasound or MRI.
But the report goes on to say...

"Your insurance may or may not cover these additional imaging studies."
Ultrasound & MRI's...
are more sensitive than mammography (i.e., higher detection rates)
But they are...
less specific, resulting in false positives, which may lead to unnecessary biopsies
So, you're one of 28M women in C & D...
The mammogram wasn't designed for your breasts...
- Resulting in low sensitivity and increasing your risk of missing cancer in the earliest stages...
Ultrasound & MRI are better...
- But these tests will cost you money and may result in frequent "scares" and unnecessary procedures that are expensive, time-consuming, risky and stressful...
THE SOLUTION
genTRU-breast
genTRU-breast is a simple blood test designed to detect breast cancer with high sensitivity and specificity (>90%), regardless of stage, age or breast density.
- genTRU B-EDT (early detection testing)*
- genTRU B-PID (post-imaging diagnostic)*
The Science
mRNA TECHNOLOGY
genTRU-breast is a novel mRNA technology that measures changes in RNA gene expression patterns found in blood to accurately detect the presence or absence of breast cancer
THE DATA
genTRU-breast is a patent-pending technology that has been extensively validated in blood from over 500 case- control clinical subjects, comprised of women from 20-80 years old across common types of breast cancer like familial, DCIS, IDC and inflammatory breast cancer, across a range of stages — including stage 0-I
Thanks,
FDA.
Opportunity for Maximum Impact
As the leading liquid biopsy company in early detection breast cancer, the new FDA rule has clearly defined the market for genTRU-breast, unlocking an opportunity for us to solve a major unmet clinical need for millions of women who are currently forced to choose between the best of two evil's — added cost and stress of additional imaging — or settling for mammography and risking their cancer going undetected.
To learn more about Mammogen and
the genTRU-breast program, send us an email.
Mammogen is owned and operated by IVBH, a global leader in liquid biopsy technology with a diversified portfolio of clinical-stage assets addressing unmet needs within women's health, pulmonary disease and metabolic health and wellness.


